Introduction to Geology
The average Oregon Caves visitor may not be able to tell stalactites and stalagmites apart, let alone comprehend the intricacies of obducted ophiolite suites. Perhaps you cannot either: that is perfectly okay, do not panic, this is a basic geology guide!
As with many sciences, geology has a lot of befuddling lingo and jargon. Indeed, it is like learning another language. Without some sort of guide to describe what we observe, interpretation would be impossible, and every process/term/idea would have to be endlessly explained. So, let this geology introduction, room-by-room guide, and glossary teach you the geo-language basics so you too can discover some of the “unusual scientific interest and importance” of the Oregon Caves.
Geology 101
Geology is the study of the Earth and its processes. The basic units of geology important at the Oregon Caves include rocks and minerals, erosion, transportation and deposition, and plate tectonics.
Rocks and Minerals
Nearly all rocks consist of minerals. A mineral is defined as a naturally occurring, solid, inorganic element or compound that has a defined chemical composition, crystal structure, and physical properties. A rock is a consolidated mixture of minerals (Tarbuck and Lutgens 697, 700). As an analogy, think of letters and words: minerals are the letters and rocks are the words.
For example, the most common mineral in the cave is calcite. Calcite has a chemical composition of CaCO3 (calcium carbonate), with a distinct crystal structure. It also has unique properties such as relative softness and being easily dissolved in acid (Bates and Jackson 94; Onac 372-373). The most common rock in the cave is marble. Marble is composed of crystals of calcite along with lesser amounts of other minerals like graphite. So, in keeping with our analogy, the minerals calcite and graphite, like letters, combine to make the rock marble, like a word.
Rocks form under various conditions and can be divided into three major classifications: igneous, sedimentary, and metamorphic. Igneous rocks can be further divided into intrusive and extrusive rocks depending on where the molten rock cooled. Extrusive rocks, such as basalt, solidified at or near the surface where they cooled relatively quickly to form small crystals usually not visible to the naked eye. Intrusive rocks, such as granite and diorite, crystallized deeper in the crust where they cooled much slower and large crystals formed (Tarbuck and Lutgens 26, 110).
Rocks weather and erode away to form sediments like boulders, sand, silt, and clay. These sediments build up over time until eventually they are compressed and cemented together to form rocks. Sandstone will form when sand grains are cemented together, just as silt will form siltstone and clay will form shale. We call these sedimentary rocks because most are composed of sediments. Other sedimentary rocks are created by precipitation from water. For example, the limestone seen in the cave is formed by calcite that crystallizes from water. Most sedimentary processes occur at or near the surface (Tarbuck and Lutgens 28, 212-214).
The final class of rocks is metamorphic rocks. These rocks form under heat and pressure that change (or metamorphose) rocks into new rocks. Metamorphic rocks are classified based on the rock from which they originate (known as the protolith, or parent rock), the intensity of the heat and pressure (metamorphic grade), and the new minerals that form (Tarbuck and Lutgens 29, 244-245). Limestone metamorphoses into marble, while shale changes from slate to phyllite to schist, and granite transforms into gneiss (Tarbuck and Lutgens 249-250, 255).
The changes a rock undergoes from one class to another constitutes the rock cycle, as seen in the figure below. Rock classifications are often distinguished by the processes that create them. These processes—like erosion, transportation, deposition, heating, and pressure—provide the connections in the rock cycle (Murck 275-276; Tarbuck and Lutgens 31).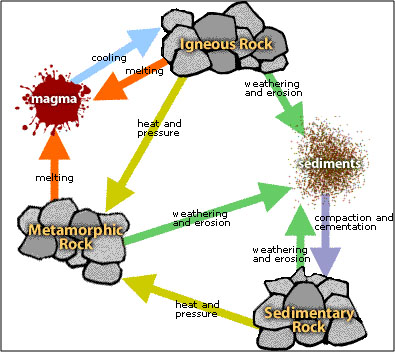
To illustrate an example of the rock cycle using this diagram, consider the following example:
Magma cools and solidifies into igneous granite->
The granite weathers into quartz sand on a beach->
The quartz sand is then cemented together into sedimentary sandstone->
The sandstone is buried deep in the Earth and metamorphosed by heat and pressure into quartzite->
The quartzite is re-melted into magma in the Earth’s mantle.
Erosion, Transportation, and Deposition
Erosion is the loosening, dissolution, or wearing away of rock, and its removal from one place to another (Bates and Jackson 222; Tarbuck and Lutgens 692). Rocks exposed near the surface encounter wind, water, ice, and living organisms; these erosional agents mechanically and chemically weather, or break apart, the rock (Tarbuck and Lutgens 703). 
Once broken, this material is transported by water or wind until it is deposited. Deposition is simply the laying down and accumulation of sediments (Bates and Jackson 175; Tarbuck and Lutgens 28). For example, the pebbles and cobbles seen along the River Styx’s streambed were weathered and eroded from rocks farther up the mountain, then transported and deposited by the stream water.
Plate Tectonics
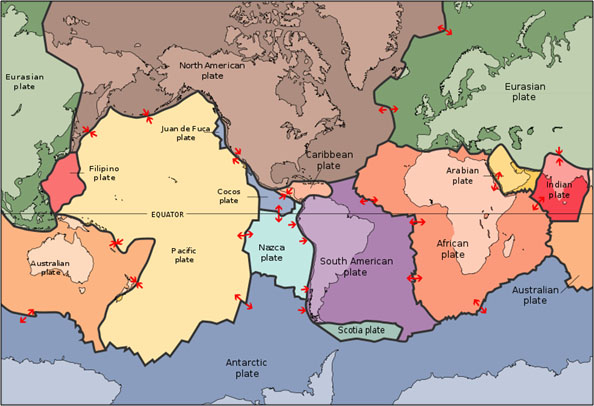
The Earth’s crust is broken into huge rock plates that more or less fit together like pieces of a jigsaw puzzle as seen in the figure above. In general, the plates are made up of continental and oceanic material. The continental material is relatively thick, less dense, and granitic. The oceanic material is relatively thin, denser, and basaltic (Tarbuck and Lutgens 60). The plates move around on top of currents in the mantle. The diagram below shows how hotter, lighter material rises and colder, dense material sinks (Tarbuck and Lutgens 69).
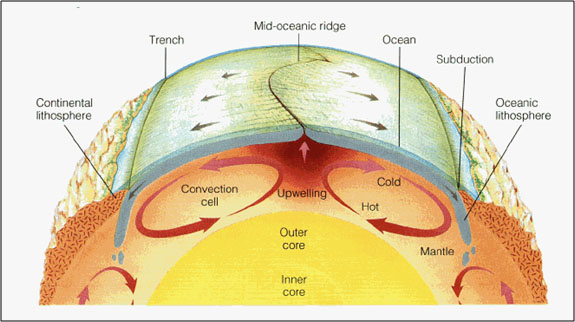
At their boundaries, these plates are spreading apart, crashing together, or sliding against one another. When two plates pull apart, new crust is formed. Generally, this occurs at mid-ocean ridges such as the Mid-Atlantic Ridge that separates the South American and African plates (Tarbuck and Lutgens 56, 57).
Some plates are coming together at their boundaries. When two plates collide, rocks are often compressed or tilted into mountain chains. For example, the Himalayas formed where the Indian and Eurasian plates converge. However, more often, ocean plates made up of dense basalt rock will be subducted, sliding underneath continental plates of less dense diorites, such as the present-day subduction of the Juan de Fuca plate underneath the North American plate in the Pacific Northwest (Tarbuck and Lutgens 56, 58; Murck 15, 17). Mid-Ocean ridges, island chains and other types of seafloors pile onto the continent likely what formed the Klamath-Siskiyou Mountains that contain the Oregon Caves.
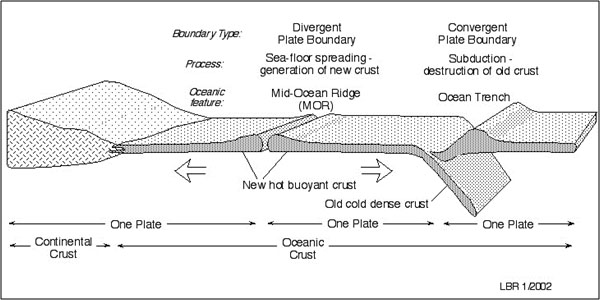
Finally, when two plates slide against one another, such as the sliding of the Pacific and North American plates at the San Andreas fault, each moves in the opposite direction. Plates move fairly slowly, at about the rate of fingernail growth on average (Tarbuck and Lutgens 56, 62, 64). However, when this movement is slowed or stopped, pressure builds. The release of pressure sends shockwaves through the rock, which are felt on the surface as earthquakes. These can happen along any type of plate boundary, but are most common along plates that are pressing against each other (Tarbuck and Lutgens 329-330, 331).
Regional Geology
Plate tectonics are the driving force in understanding the regional geology of the Oregon Caves. The geology in this region is quite complex when compared to that of other regions, but the following description provides a succinct outline of what has happened here.
The story of how the Oregon Caves formed began roughly 250 million years ago in the shallow sea floor of the Pacific Ocean when the biological activity of microbes such as cyanobacteria and possibly green sulfur bacteria began to drop crystals of the mineral calcite out of seawater. Through photosynthesis, the microbes took in carbon dioxide and caused calcite to precipitate, or become solid, out of seawater. This calcite formed the layers of a sedimentary rock, limestone.
This process continued building limestone layers on the sea floor until they were hundreds of feet thick. Successive layers of rock were then deposited on top of this limestone. The Pacific Ocean plate, which contained the limestone, continued to move eastward until eventually the rocks sank into a deepening ocean basin and were buried by sediments piled on top of them.
By the time the reef was miles beneath the sea floor, a hot seafloor thrust under it began to metamorphose the limestone, turning it into marble. The rise of nearby molten rock likely added to this process of metamorphism. In addition to metamorphism, the rocks began to uplift forming the Siskiyou Mountains. The marble was uplifted as well, until it came to its present location in the Oregon Caves National Monument, where it was exposed to the outside world. Since then, it has been subject to various forms of weathering. Some of these forms of weathering have resulted in a cave system that has since been determined by the United States Government to have "unusual scientific interest and importance."
Cave Geology
Caves are venues for understanding processes that are found nowhere else. Speleology—the study of caves—encompasses a variety of disciplines including geology, ecology, biology, exploration, history, and more. Cave geology is a particularly fascinating study because the rocks can be seen in three dimensions and most cave structures and formations are unique to cave systems. But cave geology is still a broad term: chemistry, meteorology, and many other sub-disciplines of geology all contribute to studying cave geology. Combined, these scientific studies illuminate the awe and wonder of caves.
The Oregon Caves displays both classic and unusual cave geology. Though the regional geologic history is quite unique, the actual cave development and cave formations share the timeless grandeur of an average mountain cave. However, due to its variety of sediments and rocks, Oregon Caves is markedly different from other cave systems. Sediments, ranging from stream gravels, chert pieces, and volcanic ash, can be found. All three major rock types (sedimentary, metamorphic, and igneous) are also present in the cave. These distinguishing features of the Oregon Caves are grounded in smaller-scale processes, though. All cave geology starts at the atomic level.
Cave Chemistry
In caves, all processes are based on chemical reactions. In general, there are two chemical reactions of importance: the dissolution (erosion) and the re-crystallization (deposition) of calcite. Water (H2O), carbon dioxide (CO2), carbonic acid (H2CO3), and calcium carbonate (CaCO3, or calcite) are the key chemicals involved.
Water mixes with carbon dioxide in the air and soil, which creates carbonic acid. Carbonic acid will dissolve calcium carbonate. This is because charged hydrogen atoms (H+) act like playground bullies and push around other charged atoms. One charged hydrogen ion from carbonic acid (H2CO3) will displace the calcium in calcium carbonate (CaCO3). The remaining calcium atom (Ca2+) and bicarbonate molecule (HCO3-) now carry a charge, and are called ions. These ions are dissolved in and transported by water. It is useful to consider the ions as still being calcite, just dissolved in water, like sugar or salt. This is considered an erosional process.
However, these charged particles do not stay dissolved in water indefinitely. If the chemical conditions change, the calcium and bicarbonate ions can come back together to form solid calcite. One way they can become solid again is if the water is saturated, meaning it is so full of dissolved calcite that it can dissolve no more.
Another way is if carbon dioxide escapes from the water-calcite solution, a process called degassing. Degassing changes carbonic acid (H2CO3) back into water (H2O) through the removal of carbon dioxide (CO2). Without the acid "bullying" the calcium ion (Ca2+) away from the carbonate ion (CO32-), the two ions merge to form solid calcite again (James 183). This is a depositional process.
The picture below details the basic chemical reactions taking place at Oregon Caves National Monument.
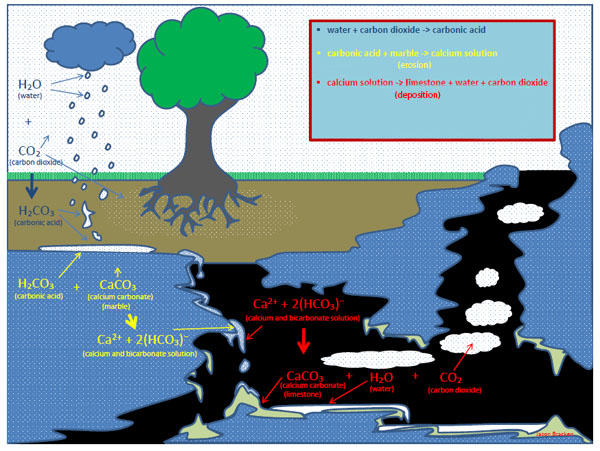
The dissolution and crystallization of calcite is a balance between the amount of acid and the amount of calcite dissolved in water. It is also a balance between the amount of carbon dioxide (CO2) in the air of the cave relative to the carbon dioxide dissolved in water as carbonic acid (H2CO3). More carbon dioxide in the air can limit degassing, which in turn limits deposition. Less carbon dioxide in the air encourages degassing, which could be one reason for faster formation growth in the Oregon Caves Exit Tunnel. Both air and water flow are important factors that influence how fast erosion and deposition occur (James 183; Dreybodt 295).
The entire cave has higher levels of carbon dioxide than what occurs outside, which can make visitors breathe more rapidly (James 183; Roth 21). On average, the cave has about double the concentration compared to the surface in winter and up to six times greater during the summer (Hale 2011).
One reason for this is the degassing of carbon dioxide from the water entering the cave. However, there is more water coming into the cave during the winter, but much less carbon dioxide. A likely reason for this is that there is relatively little biological activity going on outside during winter. Little biological activity results in less carbon dioxide available for groundwater to carry into the cave.
Cave Development
The present-day Oregon Caves likely began forming 1.6 to 1.7 million years ago (Ersek). Solution caves, like the Oregon Caves, start out as solid rock. Fractures—like joints, faults, and bedding plane partings—allowed water to move through the rock (Palmer 78 a; Palmer 440 b). The carbonic acid in the water then started to dissolve the calcite-rich rock, marble, until passageways developed. The chemical processes involved were described in the Cave Chemistry section.
While this process may seem simple, complications can occur. First, not all marble dissolves at the same rate. Second, not all water has the same concentration of carbonic acid. Third, not all rocks found inside the cave contain the same amount of calcite (Dreybodt 296-297). These complications have a drastic impact on the shape and size of cave passageways.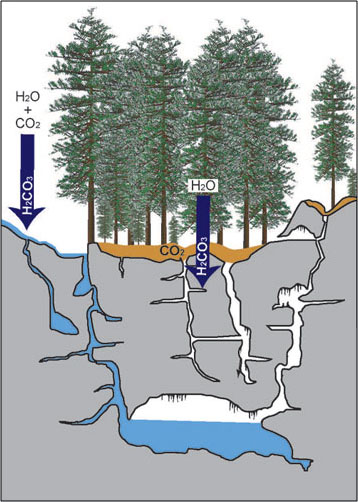
Passageways form based on how groundwater moves through the rock. For example, a passage can start forming either above or below the water table, affecting its shape and size (Palmer 90-92; White and Culver 82; Veni 436-437). Passageways formed from faults or joints below the water table tend to be wider. Groundwater collects at the water table, where it is more likely to dissolve horizontally into the marble.
The passageways between Watson’s Grotto and the Petrified Garden have sharp turns and are taller than they are wide. These formed closer to the surface, along joints (Roth 7; Palmer 78). Other passageways are rounded and are wider than they are tall, like at the Grand Column. These are much deeper under the surface and likely formed below the water table (Roth 31; Veni 438).
The erosion of passages through the marble continued until large openings to the outside world formed. This happened as late as 516,000 years ago (Turgeon). Entrances are important for formation growth because airflow changes the amount of carbon dioxide trapped inside the cave—see the cave chemistry section for more detail (Hill and Forti 692). At Oregon Caves, caused a drastic decrease of carbon dioxide from the cave, which in turn encouraged formation growth (Turgeon). Entrances also allow light and animals to enter the cave, making near-entrance zones such as the 110 Exit biologically rich areas. These areas are known as twilight zones and ecotones (Pentecost 318-319; White 219).
Not all cave development is from geologic processes. Humans can also create passageways. The Connecting Tunnel, the Exit Tunnel, and sections between Niagara Falls and the Spiral Stairs were blasted by dynamite. Because of how fast these passageways were created and their size, they needed to be managed properly in order to prevent excess airflow, ecosystem disruption, and the destruction of sensitive cave formations like soda straws (Cigna 499). Airlock doors in the Connecting Tunnel and Exit Tunnel block airflow through these artificial spaces, returning it to a more natural, "pre-dynamite" state.
Eventually, caves are destroyed. Gravity and surface processes weaken the rock, leading to collapse, and sections of the cave can get filled in with sediment that prevent erosion and deposition. But cave systems are complex. Cave development does not follow a simple linear progression. Instead, cave systems are dynamic and different places in a single cave can be developing at different stages (Palmer 440).
Caves are carved by erosion and then decorated by deposition. Formations created by erosion are called speleogens. Formations created by deposition are called speleothems (White and Culver 628). Both water and air chemistry play large roles in speleothem formation.
The concentration of carbon dioxide will always try to balance itself; chemistry shows a tendency towards equilibrium whenever possible. If there is more carbon dioxide in the water of the cave than in the air of the cave, the carbon dioxide will move from the water to the air (Hill and Forti 692). When carbon dioxide leaves the water, it reduces the water's acidity. Less acidic water can hold less calcite, so as the water loses acidity, calcite will settle out of the water in the form of crystals (Herman 613-614). Entrances introduce air with less carbon dioxide than the water, so more calcite can crystallize into cave formations (Roth 275).
Evaporation also has a limited influence on formation growth. Formation growth by evaporation of water is restricted to the areas near entrances. Dry air from outside the cave enters and causes water to evaporate, leaving behind the calcite. Evaporation of water leads to the formation of cave popcorn and other coralloids (Hill and Forti 691-692). Temperature can affect this process, since warm air can hold more moisture than cold air (Roth 8, 271).
The flow of water also affects what kinds of formations accumulate. Flowing water, dripping water, pooled water, seeping water and water condensation all create different kinds of speleothems (Hill and Forti 691). Most cave waters are supersaturated with calcite, but the rate of deposition can be quite slow (Herman 613). In the Oregon Caves, the average rate of deposition is 1 inch per 1,000 years.
However, deposition is not constant. Most of the deposition in the Oregon Caves took place during an interglacial period—a relatively warm time when the glaciers were receding and there was lots of water able to enter unfrozen ground (Ersek).
In terms of cave geology, water is a unique geologic agent because it can cause both erosion and deposition (Herman 609). Within the cave, water is everywhere. It is a part of almost every process, greatly influencing cave development and formation growth. Water delivers acid, sediments, and nutrients to cave systems. It also holds dissolved calcite, which accumulate into formations. Eventually, water finds its way out of the cave, bearing the unique chemical signature of the rock it percolated through (Fairchild and Tooth 213).
 Water takes several forms in the cave. Condensation water, pooled water, and flowing water are all visible along the tour route. One of the most notable features is the stream gushing out from the main cave entrance. The River Styx is a disappearing stream that flows underground through parts of the cave. Disappearing streams are associated with karst topography (Palmer 30). The stream has erosive power in the cave because it flows over impermeable argillite and enters the cave fairly acidic (Roth). Stream erosion is evident in the passageways between Watson’s Grotto, Petrified Garden, and Belly of the Whale.
Water takes several forms in the cave. Condensation water, pooled water, and flowing water are all visible along the tour route. One of the most notable features is the stream gushing out from the main cave entrance. The River Styx is a disappearing stream that flows underground through parts of the cave. Disappearing streams are associated with karst topography (Palmer 30). The stream has erosive power in the cave because it flows over impermeable argillite and enters the cave fairly acidic (Roth). Stream erosion is evident in the passageways between Watson’s Grotto, Petrified Garden, and Belly of the Whale.
Caves and Climate Change
Water flowing into the cave has been largely reduced in the last hundred years due to fire suppression and global climate change. As a result, formation growth has been severely reduced, and it is likely that few stalagmites have been actively growing for at least the past fifty years (Roth 28). Most formation growth occurs in the middle of warm interglacial periods, typically in the fall and spring when water is not frozen, too acidic, or absent (Roth 28).
Forest fires have been suppressed on the Monument for almost a century. As a result, more vegetation has grown on the surface. Plants absorb water from the ground and it is likely that the cave is losing water to the plants above. The water collection buckets, like the one in the Imagination Room, are measuring the amount of water flowing into the cave to test for deviations from the expected flow of water. The findings from these water collection buckets may one day result in a controlled burn that will be conducted above the cave. Returning the area around the Monument to its natural fire regime will hopefully bring more water into the cave by limiting vegetation growth (Roth 24).
Drought results from higher evaporation rates, smaller snow packs, decreased summer rain, and more vegetation as a result of fire suppression. All this removes water before it reaches the Oregon Caves. Formation thickness during past inter-glacial periods, compared both with growth in the last century and water chemistry in the last decade, shows a tenfold decrease in growth and increased solution of nearly all stalagmites.
The warming and drying of the cave can kill cave-adapted endemics made vulnerable by elongated limbs and loss of waxy coverings. Endemics can act as a "canary in the cave," as their presence and continued survival relies on consistent cave conditions. Concerns for endemic species, lower rates of formation growth, and drier conditions overall indicate that climate changes over just the past century have dramatically altered the state of the Oregon Caves.
Bates, Robert and Julia Jackson, ed. Glossary of Geology. Alexandria: American Geological Institute, 1987. 94, 175, 222.
Davies, W.E. and I.M. Morgan. “Solution cave geology.” Geology of Caves. U.S. Geological Survey Publication, 2009. Print.
Dreybodt, Wolfgang. “Dissolution: Carbonate Rocks.” Encyclopedia of Caves and Karst Science. John Gunn, ed. New York: Fitzroy Dearborn, 2004. 295-298.
Ersek, V., H. Cheng, S. W. Hostetler, F. S. Anslow, P. U. Clark, A. C. Mix, and R. L. Edwards. “Environmental influences on speleothem growth in southwestern Oregon during the last 380,000 years.” Earth and Planetary Science Letters. Elsevier. 279, 316-325.
Fairchild, Ian and Anna Tooth. “Chemistry of natural Karst Waters.” Encyclopedia of Caves and Karst Science. John Gunn, ed. New York: Fitzroy Dearborn, 2004. 213-215.
Herman, Janet. “Water Chemistry in Caves.” Encyclopedia of Caves. David Culver and William White, ed. Burlington: Elsevier Academic Press, 2005. 609-614.
James, Julia. “Carbon Dioxide-Enriched Air.”. Encyclopedia of Caves and Karst Science. John Gunn, ed. New York: Fitzroy Dearborn, 2004. 183-184.
Klimchouk, Alexander. “Caves.” Encyclopedia of Caves and Karst Science. John Gunn, ed. New York: Fitzroy Dearborn, 2004. 203-205.
Klimchouk, Alexander. “Speleogenesis.” Encyclopedia of Caves and Karst Science. John Gunn, ed. New York: Fitzroy Dearborn, 2004. 666-668.
Murck, Barbara. Geology: A Self-Teaching Guide. New York: John Wiley & Sons, 2001. 275-276.
Onac, Bogdan. “Minerals.” Encyclopedia of Caves. David Culver and William White, ed. Burlington: Elsevier Academic Press, 2005. 371-378.
Palmer, Arthur. “Cavernous Rocks: Rock Structure: Fractures.” Dayton: Cave Books, 2007. 78-80.
Palmer, Arthur. “Passage Growth and Development.” Encyclopedia of Caves. David Culver and William White, ed. Burlington: Elsevier Academic Press, 2005. 440-444.
Pentecost, Allan. “Entrance Habitats.” Encyclopedia of Caves and Karst Science. John Gunn, ed. New York: Fitzroy Dearborn, 2004. 318-319.
Roth, John. “Interpretive Manual for the Monument’s Showcave”. Cave Junction: Oregon Caves National Monument, 2011.
Tarbuck, Edward and Frederick Lutgens. Earth: An Introduction to Physical Geology. Upper Saddle River: Pearson-Prentice Hall, 2005.
Veni, George. “Passages.” Encyclopedia of Caves. David Culver and William White, ed. Burlington: Elsevier Academic Press, 2005. 436-440.
White, William. “Entrances.” Encyclopedia of Caves. David Culver and William White, ed. Burlington: Elsevier Academic Press, 2005. 215-220.
White, William and David Culver. “Caves, Definition of.” Encyclopedia of Caves. David Culver and William White, ed. Burlington: Elsevier Academic Press, 2005. 81-85.
White, William and David Culver. “Glossary.” Encyclopedia of Caves. David Culver and William White, ed. Burlington: Elsevier Academic Press, 2005. 619-629.